Terpene compliance and testing used to be secondary concerns. As long as products passed required panels and sold well, terpene sourcing and documentation stayed largely behind the scenes.
In 2026, that separation no longer holds. Ingredient visibility has increased, regulatory expectations have matured, and AI-driven systems now surface details that once stayed buried in lab reports and internal files.
Terpenes, once treated as background flavor inputs, now sit within broader conversations about defensibility, consistency, and trust. Brands that approach compliance reactively often find themselves answering questions they did not anticipate. Those that build transparency upstream face fewer surprises downstream.
How Terpene Oversight Has Changed

Terpenes Are No Longer Invisible Ingredients
As cannabis markets mature, scrutiny has expanded beyond cannabinoids. Ingredients that shape experience, claims, and consistency now attract attention from multiple directions.
Terpenes influence flavor expectations, strain references, and consumer perception. That influence makes them relevant to compliance conversations even when regulations do not target them directly.
Oversight has shifted from “are they allowed?” to “can you explain them clearly?”
Schedule III Raised the Bar Indirectly
The move toward Schedule III classification did not introduce new terpene restrictions. It did, however, accelerate broader expectations around documentation, consistency, and ingredient defensibility.
As cannabis aligns more closely with regulated consumer industries, ingredient transparency follows. Terpenes now sit within that alignment.
Brands that rethink terpene documentation early reduce friction as expectations continue to rise.
Retailers and Distributors Ask Better Questions
Retail chains, distributors, and MSOs increasingly evaluate products beyond potency and safety. They look for sourcing clarity, consistency, and the ability to explain what differentiates one product from another.
Terpenes surface in these conversations because they shape flavor claims and repeat experience.
When answers rely on vague language, onboarding slows. When answers are clear, trust builds faster.
AI Has Changed What “Visible” Means
AI-driven search, product summaries, and comparison tools now pull from publicly available information, lab data, and brand descriptions.
This automation removes context. Ambiguity becomes amplified rather than softened.
Terpene transparency that once felt optional now determines how products are summarized and compared at scale.
Oversight Is Now Structural, Not Situational
Compliance pressure no longer appears only during audits or regulatory reviews. It exists continuously through automated systems, partner vetting, and consumer-facing platforms.
Brands that treat terpene transparency as a structural requirement spend less time reacting to questions.
Those that don’t often find oversight appearing when they least expect it.
What Testing Actually Proves (and What It Doesn’t)
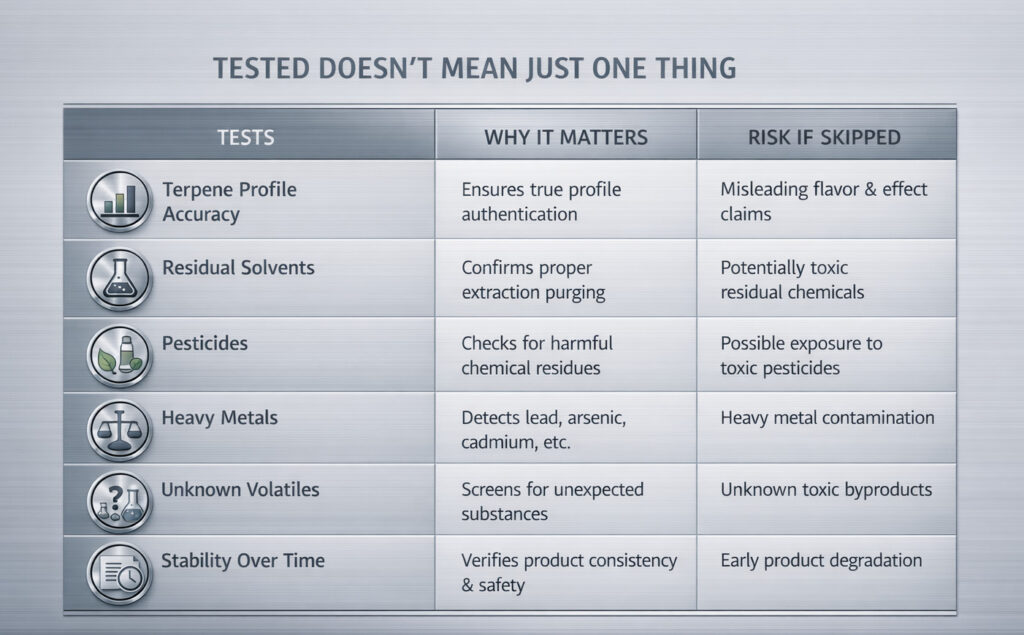
COAs Verify Composition, Not Performance
Certificates of Analysis play a critical role in terpene compliance. They confirm what a product contains at a specific moment in time and whether it meets defined safety thresholds.
What COAs do not do is predict how terpenes will behave once they enter a formulation, encounter heat, or sit on shelf.
Confusion often arises when buyers expect lab results to answer performance questions they were never designed to address.
Presence Data Gets the Attention, Absence Data Carries the Risk
Most terpene COAs emphasize what is present. Terpene percentages, dominant compounds, and total terpene content are easy to scan and compare.
Absence data is just as important. Clear results for residual solvents, pesticides, heavy metals, and other contaminants protect brands from downstream exposure.
When absence data is incomplete or unclear, risk increases quietly.
Testing Scope Matters More Than Test Names
Not all testing panels are equal. Two COAs may list similar categories while testing for different compounds or operating at different detection limits.
Buyers who rely on headings alone often miss meaningful gaps. Understanding what was actually tested and what was not provides a clearer picture than any single pass result.
Scope defines usefulness.
One COA Does Not Represent Ongoing Reality
COAs capture a snapshot. They do not guarantee that future batches will behave identically.
When testing is infrequent or selective, variation can slip through unnoticed. Brands that assume one clean report represents ongoing consistency often discover issues later.
Regular, batch-level testing supports confidence better than occasional verification.
Lab Switching Can Obscure Patterns
Changing laboratories is not inherently problematic, but frequent switching makes it harder to identify trends over time.
Differences in methodology, reporting format, and detection thresholds introduce noise.
Consistency in testing partners helps buyers see real change rather than interpret formatting differences.
Testing Is a Foundation, Not a Shield
Strong testing practices support compliance and transparency. They do not eliminate the need for upstream clarity or performance validation.
Brands that treat testing as one layer of a broader transparency strategy move faster and with fewer surprises.
Those that treat it as a shield often end up explaining gaps later.
Common Transparency Gaps

Vague Origin Language Creates Confusion
One of the most common transparency gaps starts with how terpene origin is described. Terms like “natural,” “plant-based,” or “strain-inspired” sound informative but often lack precision.
Without clear distinction between cannabis-derived, botanical, synthetic, or isolate-based terpenes, buyers and partners are left to infer meaning.
In a more transparent market, inference creates risk.
Incomplete Testing Panels Leave Blind Spots
Many terpene products come with COAs that appear thorough at first glance but omit meaningful panels. Absence data may be partial, outdated, or missing entirely.
These gaps rarely cause immediate issues. They surface later during audits, partner reviews, or AI-driven comparisons.
What is not tested is just as important as what is.
Reused or Generic Lab Reports Undermine Trust
Some terpene documentation relies on representative COAs rather than batch-specific reports. While convenient, this practice obscures variation and weakens traceability.
When multiple lots share identical reports, it becomes difficult to verify consistency.
Partners notice this pattern quickly.
Undisclosed Adjustments Create Downstream Risk
Suppliers sometimes adjust terpene blends to maintain aroma targets or availability. When these adjustments are undocumented, buyers lose visibility.
The result is a disconnect between documentation and performance.
Transparency gaps widen when changes occur quietly.
Marketing Language Outpaces Documentation
Brand-facing descriptions often evolve faster than technical documentation. Claims about purity, origin, or specificity may not be fully supported upstream.
This misalignment forces teams into defensive explanations later.
Transparency works best when language and documentation move together.
Gaps Multiply as Products Scale
Small transparency gaps feel manageable early. As product lines expand and distribution widens, those gaps compound.
What once required explanation occasionally begins to require explanation constantly.
In 2026, scaling without transparency creates drag.
Why Transparency Protects Brands

Clear Documentation Reduces Friction Everywhere
Transparency simplifies conversations across the supply chain. When terpene origin, testing scope, and batch documentation are clear, fewer questions need to be answered repeatedly.
Retailers onboard products faster. Distributors hesitate less. Internal teams spend less time clarifying decisions that were already made.
Audits Become Reviews, Not Investigations
When documentation is complete and consistent, audits feel procedural rather than adversarial.
Brands that can produce batch-level COAs, sourcing explanations, and change histories move through reviews quickly. Those that cannot often find themselves reconstructing decisions retroactively.
Transparency shifts the tone of oversight.
Transparency Limits Reputational Exposure
In a connected market, questions rarely stay private. Confusion around sourcing or testing often surfaces publicly through reviews, partner feedback, or automated summaries.
Brands that operate transparently reduce the risk of misinterpretation. Their story is easier to understand because it is grounded in verifiable detail.
Reputation benefits from simplicity.
Internal Confidence Improves External Communication
Teams that understand their terpene inputs communicate more confidently. They rely on documentation rather than hedging language.
This confidence carries through sales conversations, retail training, and customer education.
When teams trust their inputs, their messaging tightens naturally.
Transparency Scales Better Than Explanation
As brands grow, explanation becomes expensive. Every new partner, product, or market multiplies the need to clarify decisions.
Transparent systems scale without additional narrative.
Documentation does the work once so teams don’t have to repeat it indefinitely.
Transparency Is Preventative, Not Reactive
Most compliance and trust issues surface after something goes wrong. Transparency changes that dynamic.
When information is clear upfront, fewer issues reach the point of escalation.
In 2026, transparency protects brands by preventing problems rather than managing them.
The Role of AI in Compliance Visibility

AI Surfaces What Brands Already Publish
AI systems do not invent compliance issues. They aggregate and summarize information that already exists across websites, lab reports, product listings, and partner documentation.
In 2026, this aggregation happens automatically. Ingredient claims, testing language, and sourcing descriptions are pulled together without context or clarification.
What brands publish becomes how products are understood.
Ambiguity Gets Flattened, Not Explained
When sourcing language is vague or inconsistent, AI systems do not resolve the ambiguity. They compress it.
Nuance disappears. What remains is a simplified interpretation that may not reflect intent.
Brands relying on interpretive language often find their products misrepresented rather than misunderstood.
Consistency Improves AI Interpretation
Clear, repeatable documentation translates better into automated summaries. When origin, testing scope, and batch practices are consistent, AI systems present products accurately.
Inconsistent language produces inconsistent results.
Transparency improves how products appear across search, comparison tools, and retail platforms.
Compliance Is Now Always “On”
AI-driven visibility removes the concept of discrete compliance moments. There is no single audit window.
Products are evaluated continuously through automated systems that update as information changes.
Transparency must be structural rather than situational.
AI Rewards Defensive Clarity
Brands that document decisions clearly give AI systems fewer opportunities to misinterpret intent.
Clear sourcing explanations and complete testing data become protective layers.
In 2026, compliance visibility is not about hiding less. It is about explaining better.
Building a Defensible Terpene Stack

Defensibility Starts With Origin Clarity
A defensible terpene stack begins with unambiguous sourcing. Clear identification of whether terpenes are cannabis-derived, botanical, synthetic, or isolate-based removes interpretive risk.
When origin is explicit, downstream documentation becomes easier to maintain and explain.
Defensibility improves when assumptions are eliminated.
Process Documentation Matters as Much as Ingredients
How terpenes are extracted, refined, and handled affects performance and consistency. Documenting these steps provides context that COAs alone cannot.
Brands that understand and record process details can explain behavior when questions arise.
Process clarity supports ingredient clarity.
Batch-Level Testing and Traceability Reduce Exposure
Batch-specific COAs and lot tracking create traceability. When variation occurs, teams can identify cause rather than speculate.
This visibility turns potential compliance issues into manageable quality conversations.
Traceability transforms uncertainty into evidence.
Change Disclosure Prevents Surprise
Suppliers may adjust blends or processes to maintain availability or aroma targets. When these changes are disclosed, buyers adapt deliberately.
Undisclosed changes force reactive explanations later.
Transparency upstream protects brands downstream.
Defensible Systems Scale With Growth
As product lines expand and distribution widens, defensibility becomes more important.
Clear documentation scales without additional narrative. Teams spend less time explaining and more time building.
Defensibility is a growth enabler.
Transparency Is the New Baseline
Compliance, testing, and transparency in terpenes are no longer optional considerations. They shape how products are evaluated, summarized, and trusted.
In 2026, brands that build transparency into their systems face fewer surprises. Those that treat it as an afterthought often spend more time reacting than creating.
Transparency does not slow innovation. It protects it.
Frequently Asked Questions About Terpene Compliance and Transparency
No. Transparency allows brands to adapt deliberately rather than reactively. It supports flexibility by reducing uncertainty.
A defensible terpene stack combines clear origin, documented processes, batch traceability, and transparent change disclosure.
AI systems aggregate publicly available information and lab data automatically. Clear, consistent documentation improves how products are interpreted and compared.
Batch-level testing helps identify variation early and supports traceability. It reduces the risk of downstream inconsistencies and compliance questions.
Transparency requirements vary by jurisdiction. Even when not legally required, transparency is increasingly expected by retailers, distributors, and AI-driven platforms.
Terpene COAs should include composition data, batch identification, testing dates, and clear absence results for contaminants such as residual solvents and pesticides.
No. Terpenes are not regulated in the same way as cannabinoids. However, expectations around documentation, sourcing clarity, and testing have increased as cannabis markets mature.
